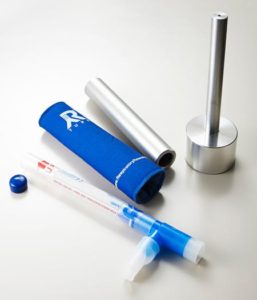
RTube™
Breath Condensate Collection Device
Product Features
- Over 400 published studies performed using RTube
- Reproducible, reliable results in over 230,000 collections
- Single-use disposable device eliminates infectious cross-contamination and cleaning
- Sealable condensation tube is easily mailed enabling centralized sample analysis
- Syringe-style plunger efficiently sweeps condensate from the tube walls into a fluid pool for easy analysis
- Saliva trap separates saliva from the exhaled breath and prevents it from entering the condensation tube
- Exhalation valve enhances condensation efficiency and produces high condensate volumes
- Simple handheld device is easy to use by the unsupervised subject at home
Overview
Portable Collection Devices for the Study of Respiratory Droplets in Exhaled Breath Condensate
Exhaled Breath Condensate (EBC) is composed of droplets of Airway Lining Fluid (ALF) evolved by turbulence from all lung compartments and held in a matrix of condensed moisture from the breath. These droplets contain numerous breath biomarkers including DNA, RNA, mRNA, proteins, metabolites, and volatile organic compounds (VOC). The RTube™ Exhaled Breath Condensate collection device captures these droplets and presents them as a fluid pool which can be easily extracted and analyzed.
The RTube is designed for ease of use even by the unsupervised patient in the home, workplace, laboratory, hospital, or clinic. This non-invasive handheld device is fully self-contained and disposable. As the subject breathes normally into the device, the RTube gathers breath condensate in a transportable/mailable cartridge. Simplicity of use allows for easy integration of the RTube into existing studies and allows large amounts of EBC data to be collected with ease from subjects in the clinic, hospital, home, workplace, school, or any other reasonable environment.
Our special valve assembly is the key to efficient sample collection and allows unsupervised subjects to easily gather and store contaminant-free samples. Typical condensate fluid yield is 100 microliters/minute for a child and 200 microliters/minute for an adult at normal tidal breathing effort.
 The unique one-way valve provides maximal particle impaction on the condensing surface and also acts as a plunger. The ability to pool the sample near the upper end of the tube allows for maximum sample recovery in seconds.
The unique one-way valve provides maximal particle impaction on the condensing surface and also acts as a plunger. The ability to pool the sample near the upper end of the tube allows for maximum sample recovery in seconds.
The RTube collection system utilize inert, FDA-approved polypropylene material for minimal water absorption. The blue one-way valve is crafted from FDA-approved silicone rubber. End caps made of medical grade vinyl seal the sample for long-term storage. A label is also provided allowing the researcher/subject to record the name and date of collection. Each RTube is assembled and cleaned to exacting specifications, placed in an individual sealed pouch, and bulk packaged for delivery to you. For your convenience, we offer several Specialty Kits designed to fit the most common needs of our customers. Let us help you select the RTube Specialty Kit that best fits your needs.
 Cooling Sleeve
Cooling Sleeve
The cooling sleeve is necessary for efficient condensate collection. Usually stored in a laboratory freezer until needed, the sleeve is designed to easily slide over the RTube and create the perfect environment for condensate collection. An Insulating Cover is included with every Cooling Sleeve.
Our high quality cooling sleeves are cold-worked from solid aluminum and have been designed for repeated use under the harshest freeze/thaw conditions.
 Standard Plunger
Standard Plunger
Made of solid aluminum, this is a stand alone device that is necessary to utilize the syringe style plunging built into the RTube. The one way valve easily slides along the polypropylene walls and pools the sample near the top of the tube for easy removal.
How to Use
Exhaled breath condensation is a non-invasive method of gathering exhaled breath samples useful for assessing a number of disease parameters. The RTube is designed to provide maximum sample collection in minimal time. Exhaled air is directed through a unique one-way valve and into a cooled collection chamber where vapors, aerosols and moisture in the breath condense along the protected inside walls of the RTube. The one way valve is then used as a plunger that collects droplets stuck to the inside wall and holds the sample near the top of the RTube.
RTube EBC Collector and Accessories
The RTube is designed as a disposable, single use item. The cooling sleeve, the insulating cover and the plungers are fully reusable. A key feature is the ability to collect the condensate sample, store the sample, transport the sample, and analyze the sample all in the same RTube. No transfer of condensate fluid into another device is generally required, minimizing complexity in the lab and at the patient’s home. Opportunities for contamination are virtually eliminated and labor required for sample processing is minimized. Every step has been painstakingly thought out and this design created to minimize the effort and complexity for the patient, researcher, and lab technician. The system is designed for ease of use even in very large clinical studies.
Typical condensate fluid yield is 75-150 microliters/minute for a child and 100-250 microliters/minute for an adult at normal tidal breathing effort. Two minutes of normal breathing will yield sufficient fluid quantity for most laboratory tests, however a 7-10 minute collection time is commonly employed so that extra sample might be frozen for other investigations.
These samples can be easily collected unsupervised in the subjects’ homes or worksites and naturally lend themselves to occupational medicine and toxicology research.
The typical 5-step collection process is shown below with some details left out for clarity. Please read the last section of this manual for cautions, and general assay information.
The instructions below detail each of the four steps:
Step 1: Condense Exhaled Breath using the RTube.
Step 2: Store/Transport Condensate
Step 3: Use the Plunger to Collect EBC into a Pool at the Top of the RTube
Step 4: Analyze the Sample
Step 1: Condense Exhaled Breath Using the RTube
1-1 Prepare the cooling sleeve before taking samples:
- Place the aluminum sleeve into a plastic bag to prevent moisture from freezing to the inside of the sleeve and contaminating the sample.
- Place the sleeve into a freezer of the appropriate temperature, depending on the exhaled substance of interest. Home freezers work well for stable compounds.
- Allow the sleeve to cool to the appropriate temperature. While transporting the sleeve over long distances, store in an ice chest filled with ice or frozen packets.
1-2 Prepare the RTube: The RTubes are sent ready to use for most applications; however, when studying compounds in EBC that are found diffusely in the laboratory setting as contaminants, extra precautions should be taken. These include, but are not limited to, nitrate and nitrite. We recommend that you wash the condensing chamber with ultrapure deionized water which meets or exceeds specification ASTM D 1193 for Type I and type II Reagent Grade Water and allow them to dry fully before use to eliminate any possible contamination.
1-3 Fill out label located on the side of the collection Tube and note the location of the red arrow located on the label as this should be pointing up during the collection.
1-4 Place the blue insulating cover over the aluminum cooling sleeve to protect your hand and keep the aluminum cold.
1-5 Place the aluminum sleeve over the outside of the collection chamber.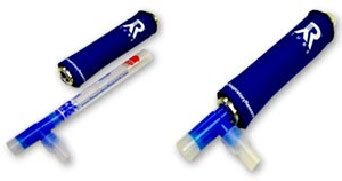
 1-6 The patient should immediately begin breathing in and out through the mouthpiece with the red arrow pointing upward. In some cases patients will have a very shallow breathing pattern and should be encouraged to exhale hard enough to “hear their breath flowing through the top of the RTube.” The one way valve will direct the exhaled air through the cooling sleeve where the sample will be collected. After extended periods of storage, the RTube may present some resistance upon the initial exhalation attempt. This is normal and easily corrected using the pre-use verification procedure here.
1-6 The patient should immediately begin breathing in and out through the mouthpiece with the red arrow pointing upward. In some cases patients will have a very shallow breathing pattern and should be encouraged to exhale hard enough to “hear their breath flowing through the top of the RTube.” The one way valve will direct the exhaled air through the cooling sleeve where the sample will be collected. After extended periods of storage, the RTube may present some resistance upon the initial exhalation attempt. This is normal and easily corrected using the pre-use verification procedure here.
1-7 Collection times should be standardized for each application. We recommend a five to seven minute collection time for most applications.
1-8 After sample collection has been completed and the sample stored properly, the patient should dispose of the mouthpiece and place the cooling sleeve back into the protective bag and refreeze.
Step 2: Store/Transport Condensate
2-1 Detach mouthpiece and discard. Place the cap on the end of the RTube opposite the blue duckbill valve and near the red arrow. Optionally, the opposite end can be also capped in addition to the end with the red arrow. Additional caps are available by request, please call for more information.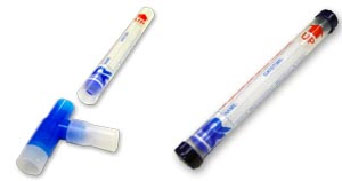
Freeze the sample at the appropriate temperature or leave at room temperature if appropriate for the compounds of interest. When studying stable compounds a sample can be stored at room temperature or in a home/laboratory freezer until needed. If packaged properly and kept out of high heat, the sample can be shipped by mail with no contamination or loss of sample fluid. In fact, the RTube is so simple to use that the unsupervised subject should be able to perform all sampling from their hospital bed, at their workplace, or from the comfort of their own home. When studying common laboratory contaminates the sample should be removed from the collector and stored in the freezer as soon as possible.
Be sure to remove the cap on the lower end (opposite the red arrow) if so equipped PRIOR to thawing. The RTube incorporates a pressure relief to ensure sample protection during the thawing process which will only function if the lower cap is removed. Keep the upper cap in place until sample is fully thawed and ready for analysis.
Step 3: Use the Plunger to Collect EBC into a Pool at the Top of the RTube
 3-1 In the laboratory, remove the cap and pool the sample by plunging the one-way duckbill valve toward the top of the Tube for easy collection. Simply place the RTube over the top of Standard Plunger and push down until the RTube touches the base of the Standard Plunger. Ensure the red arrow marked “UP” is properly oriented during this process. As the RTube is pushed down over the Plunger the nose on the end of the Plunger engages the duckbill valve and strokes it the entire length of the condensation surface much like a syringe. The result is a neatly presented .5 ml – 1.5 ml pool of pure EBC ready for analysis.
3-1 In the laboratory, remove the cap and pool the sample by plunging the one-way duckbill valve toward the top of the Tube for easy collection. Simply place the RTube over the top of Standard Plunger and push down until the RTube touches the base of the Standard Plunger. Ensure the red arrow marked “UP” is properly oriented during this process. As the RTube is pushed down over the Plunger the nose on the end of the Plunger engages the duckbill valve and strokes it the entire length of the condensation surface much like a syringe. The result is a neatly presented .5 ml – 1.5 ml pool of pure EBC ready for analysis.
Syringe-style plunger pools condensate near the top of the RTube for easy sample collection
Step 4: Analyze the Sample
4-1 This step is of course dependent on the substance of interest. The substance my be unstable, and require rapid analysis, or it may be very stable (as is the case for pH) and be analyzed anytime.
The RTube is designed as a disposable, single use item. The cooling sleeve, the insulating cover and the plungers are fully reusable.
A key feature is the ability to collect the condensate sample, store the sample, transport the sample, and analyze the sample all in the same RTube. No transfer of condensate fluid into another device is generally required, minimizing complexity in the lab and at the patient’s home. Opportunities for contamination are virtually eliminated and labor required for sample processing is minimized. Every step has been painstakingly thought out and this design created to minimize the effort and complexity for the patient, researcher, and lab technician. The system is designed for ease of use even in very large clinical studies.
Regulatory
USA: FDA Regulatory Status of the RTube family of products
RTube is registered with the United States Food and Drug Administration as a Class I device for Research Use Only. You may see our device registration at:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm?lid=198613&lpcd=KGK
As such all use of the RTube must be conducted under the supervision of an Investigational Review Board (IRB), which is common to almost all human studies, and no therapeutic or medical decisions may be based on the outcome of analyses using the RTube collector.
Europe: The RTube falls outside the EU IVD Directive and requires no CE mark
The RTube Exhaled Breath Condensate Collector is clearly and permanently labelled “For Research Use Only” on the device itself, is not an In-Vitro diagnostic (IVD), and therefore falls outside the EU IVD Directive and requires no CE mark. As such all use of the RTube must be conducted under the supervision of an Investigational Review Board (IRB), which is common to almost all human studies, and no therapeutic or medical decisions may be based on the outcome of analyses using the RTube collector.
Please see references below:
Recital 8 of the IVD Directive 98/79/EC states: “Whereas instruments, apparatus, appliances, materials or other articles, including software, which are intended to be used for research purposes, without any medical objective, are not regarded as devices for performance evaluation.”
Further based on guidance document MEDDEV. 2.14/2 rev.1 Section 5 (a):
- Potential Situations where “RUO” Labeled Products could be used
The following are a list of possible situations where RUO products could be used and which therefore fall outside the scope of the IVD Directive.
(a) RUO products used for Basic Research:
These are products used for research conducted to study all aspects of human life in an attempt to better understand all underlying mechanisms. In such studies / experiments animal and / or human models are used. No medical purpose is defined, as the specimens taken are not being used for the purpose identified in the definition of an IVD device in the IVD Directive, article 1 2(b). In such practice there is no potential to misuse RUO products.
Institutional Review Board/ Human Investigation Committee Consideration of the RTube family of products
Over 230,000 samples have been collected from ambulatory subjects worldwide with the RTube over the last 25 years with zero adverse events attributed to its use. Our device and methods have been considered non-significant risk by multiple Institutional Review Boards. Each individual Institutional Review Board/ Human Investigation Committee may wish to make its own determination as to the non-significant risk nature of the device, especially if the device is modified or incorporated into other equipment.
Technical Information
How it Works
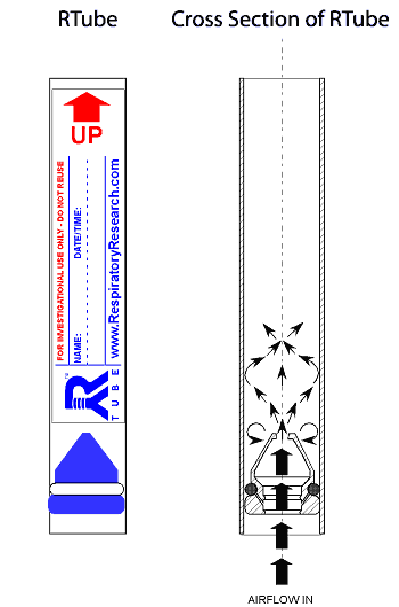 The RTube is strictly a non-rebreathing device and utilizes two one-way valves to maintain unidirectional flow throughout the entire breathing cycle as the subject inhales and exhales through the mouthpiece. As the subject breathes normally through the device, saliva is separated from the main exhalate stream in the blue “Tee” section of the mouthpiece and directed downward with the assistance of gravity into the saliva drain. From here the saliva exits the device through the one-way disc valve at the bottom of the “Tee” section. The exhaled breath stream turns upward and passes through a one-way duckbill valve into the gray condensation tube. The peak of this duckbill valve is a long, thin slit which acts as a nozzle.
The RTube is strictly a non-rebreathing device and utilizes two one-way valves to maintain unidirectional flow throughout the entire breathing cycle as the subject inhales and exhales through the mouthpiece. As the subject breathes normally through the device, saliva is separated from the main exhalate stream in the blue “Tee” section of the mouthpiece and directed downward with the assistance of gravity into the saliva drain. From here the saliva exits the device through the one-way disc valve at the bottom of the “Tee” section. The exhaled breath stream turns upward and passes through a one-way duckbill valve into the gray condensation tube. The peak of this duckbill valve is a long, thin slit which acts as a nozzle.
Turbulence is created by the nozzle as the air jet spreads out into the cylindrical condensation chamber. The asymmetric placement of the air jet within the condensation chamber directs proportionally heavier flow to hit the cylindrical surfaces parallel to the plane of the diagram below. The imbalance of flow and pressure redirects the jet to form vortexes which periodically mix. This ensures maximum impaction of suspended particles with the tube wall and greatly enhances the heat transfer from the exhalate to the tube.
The effect is easy to test: during use place your hand above the top of the RTube as you exhale. Your exhaled breath will feel cool. Remove the duckbill valve, re-freeze the cooling sleeve, and try again. This time your breath will feel warm even though you are using a fully-chilled cooling sleeve. The difference is the absence of turbulence normally created by the duckbill valve. Warm exhaled breath means lower heat transfer, less condesation, and therefore less sample yield. While many homebuilt devices can be found in the literature, none of them will yeild the efficiency of time and quality of sample you will get with the RTube.
Quality
Our commitment to quality extends far beyond our product. At Respiratory Research, Inc., it is a way of business. We require that leading industry quality practices be applied to all products and processes. This includes Supplier Qualifications, Engineering, Procurement, Manufacturing, Order Fulfillment, and Technical Support.
We work with ISO 13485-certified suppliers only. Top-tier suppliers are provided current engineering documentation at all times and apply their own internal quality systems to ensure that all products supplied to Respiratory Research, Inc. are in full conformance with these engineering specifications. Respiratory Research, Inc. thoroughly and completely documents all product requirements using engineering drawings bills of material, assembly procedures, environmental specifications, and component specifications. These are maintained by Respiratory Research, Inc. and are under full revision control.
RTube Materials and Performance
The Rtube Exhaled Breath Condensate Collector is specifically designed to meet the needs of a wide range of subjects, investigators, and clinicians. Its size, weight, materials, and performance characteristics have been carefully selected to provide the maximum safety, effectiveness, and flexibility.
| Physical Specifications | Rtube w/Cooling Sleeve (Configured for Use) | Rtube Transportable Cartridge |
|---|---|---|
| Envelope Dimensions | 29.0 cm Tall X 3.5 cm Wide X 10.0 cm Long | 22.2 cm Tall X 2.6 cm Wide X 2.6 cm Long |
| Weight | 442 grams | 17 grams |
| Material Specifications | Rtube | Cooling Sleeve |
|---|---|---|
| Mouthpiece | Polyethylene | N/A |
| Tee | Polyethylene | N/A |
| Check Valve | Polyethylene housing, Polyisoprene Rubber disk | N/A |
| Tube | Copolymer Polypropylene | N/A |
| Duckbill Valve | Silicone Rubber (FDA-approved Ingredients) | N/A |
| Oring | PTFE (Teflon) | N/A |
| Endcaps | Medical-grade Vinyl | N/A |
| Label * | Mylar | N/A |
| Sleeve * | N/A | Aluminum 6061-T6 |
| Insulator * | N/A | Polyester / Cotton Fabric with Polyester Insulation |
NOTE: Parts annotated with (*) do NOT contact condensate
| Performance Specifications | Adult | Child |
|---|---|---|
| Duration of Collection | 7 Minutes | 10 Minutes |
| Volume of Condensate Collected | 1000 microliters | 700 microliters |
| Collection Temperature | -20C | -20C |
| Flow Resistance | 0.20 cm H2O/liter | |
Cautions and Warnings
A WARNING, if unheeded, could potentially affect the patient.
A CAUTION, if unheeded, could potentially affect the quality of the sample or present a hazard to the medical and laboratory staff.
Respiratory Research has identified circumstances in which the RTube™ Exhaled Breath Condensate Collector may block exhalation. There are no safety risks to the conscious patient as he will naturally cease attempting to exhale through the device; however, an unconscious or intubated patient will be completely dependent upon external intervention to detect and correct obstruction within the device. This complete dependence upon external intervention introduces risks and requires strict attention and extra precautions.
WE RECOMMEND THE RTUBEVENT™ FOR USE WITH MECHANICALLY-VENTILATED PATIENTS. This product can be viewed here.
Respiratory Research has identified five conditions which could obstruct airflow through the RTube. These are unlikely but worthy of mention and include:
- Improper assembly of the device by the customer attempting to rebuild and re-use
- User error in applying the end caps prior to use instead of after use
- Entry of foreign material into the device after removing from factory-sealed pouch
- Light sticking in the duckbill valve
- Improper mating of condensation chamber with the mouthpiece.
Several of these items are mitigated by following the Cautions listed in the next section.
The RTube is a disposable device designed for single use. It is assembled to stringent specifications and cleaned under strictly controlled clean-room conditions to ensure proper operation, safety, and cleanliness. Any subsequent rebuilding or tampering with the device will reduce the effectiveness of the device, risk the quality of your data, and potentially pose a safety risk to the patient.
The RTube cartridge is supplied with one end-cap which should be placed on the end opposite the blue one-way valve (near the red arrow). Once the cap is properly attached, the EBC sample will be trapped inside of the collection chamber and can be stored and transported as needed. Failure to cap the correct end of the cartridge may result in sample loss during transport.
The blue one-way valve located inside of the RTube’s collection chamber is designed to relieve pressure buildup during freeze/thaw cycles via a small hole at the valve’s peak. The position and the size of the hole will prevent any loss of sample; however, during this process some gas exchange may occur. If gas exchange is a concern to your particular project we offer additional caps upon request. However, capping both ends of the tube instead of only one will disable the pressure relief mechanism.
With the pressure relief disabled, either end cap may pop off the RTube cartridge during thaw from deep freeze. This occurs due to the pressure increase from expansion of the air and vapors inside the cartridge as the temperature is normalized to ambient. This pressure buildup can pop off caps with enough force to become airborne and sample loss may occur.
Please contact RRI for more information regarding the safe and effective use of dual caps.
Nitric oxide can diffuse through various materials, and its oxides, including nitrite and nitrate, are common and abundant contaminants on laboratory surfaces.
If nitrogen oxides are to be assayed we strongly recommend rinsing with deionized water all apparatus that will come in contact with the exhaled breath condensate sample as close as possible to the time of use. This includes the inside of the RTube condensing chamber, microcentrifuge tubes, and pipette tips. Note that many latex gloves are highly contaminated with nitrogen oxides. We recommend plunging and removing sample from the RTube within 24 hours if nitrogen oxides are of interest. Additionally, we recommend analyzing the sample as soon as possible if nitrite is of interest.
The RTube is an investigational device only and is designed for research purposes. Due to the non-invasive nature of the device, the RTube is exempt from the Investigational Device Exemption of the United States Food and Drug Administration. Any invasive use of the device or use outside of protocols approved by local Institutional Review Boards is not supported by Respiratory Research, Inc.
The RTube should be handled with a slow, steady, controlled motion when inserting onto plunger. This steady motion should be maintained even if unpredictable resistance levels are met. If excessive force is used and the resistance unexpectedly drops, the rapid motion of the RTube as it is forced over the plunger can distort the internal parts and can result in partial loss of sample
Prior to use, please check to ensure the red arrow at the end of the collection chamber is pointing AWAY from the blue mouthpiece assembly.
The RTube collection chamber houses one of the two internal one-way check valves and must be properly oriented when attached to the mouthpiece. This collection chamber is easily identified as the long semi-clear polypropylene tube sitting atop the blue mouthpiece assembly. By design, these parts can easily be separated to allow isolation and transport of the sample within the chamber. The initial mating is done at the factory and quality checked to ensure correctness. However, during handling by patients or medical staff they can become separated. This is not a problem as long as they are mated correctly prior to use.
Note: The word “UP” is printed in red letters beneath the red arrow and pertains to the overall device orientation during use, not the assembly of the collection chamber to the mouthpiece.
This resistance is due to minor adhesion internal to the duckbill valve. The duckbill valve is made of silicone rubber. Silicone rubber, especially at warm temperatures, is a naturally tacky material. We minimize this tackiness through a curing process ensuring all volatiles are removed and that all residual reactions of the two-part reagent chemical mix used to create the compound are complete.
Even with this cure, the area around the slit of the valve may still experience light sticking when the RTubes are stored for longer than 30 months or stored in warm ambient temperatures.
This sticking is expected and normally has no effect, but in rare cases it can be enough to impair usage of the RTube. Apply the procedure shown here (PDF) prior to using RTube to verify proper operation and eliminate any potential impairment. Please Note: It is important that all staff handling the RTube be made aware of this procedure.
